eIRB Guidance and Videos for Member Review
In this section, we are providing guidance documents to our IRB members. If you have any suggestions to add or modify documents in this section, please email the IRB QA and Education Team.
Important Information about the system
* The new system does not prevent you from being both a reviewer and on the study team.
Our IRB staff will do their best to avoid this, but please be aware that a review could come to you in error – just let the analyst know you cannot review it.
* Error Messages when doing "Review Study" (or "Review Modification/CR" etc)
- When you click "Review Study" (or "Review Modification/CR" etc), you are presented with the smartform for the submission.
- After each "page" of the smartform, there is a checkbox for you to confirm that you've reviewed that section. After checking a couple of these, the system gives you an ERROR message.
- What to do: Click OK on the error messages, and just keep going. Or, just scan down the smartform pages and ignore the checkboxes - checking them is not required.
- Then, click "EXIT" at the end to return to the submission workspace.
- Full Board Review:
- Request clarifications if needed and if you don't mind the study team knowing who you are (use the "Request Clarifications by Committee Member")
- Otherwise just use "Add Reviewer Comments" to either make comments or say you've completed your review.
- Also please log a private comment or email the analyst if you have concerns that can be addressed before the meeting. (You can also reach out to the study team by email or phone if you think best.)
- Designated/Expedited Review: Submit your Designated Review to either approve, or request modifications.
- Full Board Review:
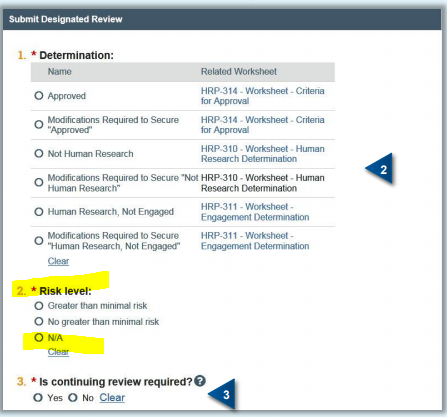
* Risk Designation on Designated Review form
When submitting a designated review, please do not select N/A under question 2 (Risk Level). N/A may indicate the study itself is not human subjects research. Please, select no greater than minimal risk if you are reviewing that type of study.
* Quick guide for reviewers: IRB Reviewer's Quick Reference (PDF)
A step-by-step graphical reference for reviewers that includes finding review checklists, viewing the study details, and submitting review decisions.
Inbox, Workspace, and Workflow Overview
Committee Review/Requesting Clarifications
Submitting Review Comments
Request Modifications