Reportable Information
Emory Studies reviewed by External IRBs
This page covers Emory and VA reporting. For studies reviewed by another IRB, please see our Collaborative and Multi-site Research.
Note: Some types of events are considered "egregious" and will still require reporting to the Emory IRB.
Emory (Non-VA) Guidance and Forms
Find out if your event is reportable by using this tool with decision trees - Chart guidance (PDF)
Reporting Obligations for Investigators (PDF) - What you need to know about what to report and when including:
- Internal and External Unanticipated Problems, Serious Adverse Events, and Deaths
- Protocol Deviations (only internal ones are reportable)
- Noncompliance with laws, regulations, Emory HRPP policies, and procedures, or the requirements of the IRB
Regardless of PI assessment, the following internal deviations are always reportable to the IRB:
- Deviations involving errors during eligibility process that caused the enrollment of an ineligible subject
- Missed protocol-required labs or procedures indicated before study intervention, including pregnancy tests (even if harm did not occur)
- REMS requirements deviations
- Drug dosing errors involving safety concerns (for example, if a subject was dosed incorrectly at a lower or higher dose, or if the drug was not stored per manufacturer indications)
- Consent process errors (for example, when subjects did not receive an adequate explanation of study, or there is no correct documentation of consent)
- Noncompliance is always promptly reportable, but feel free to check with the IRB if unsure whether your situation meets the definition of noncompliance
Timing of Report of Internal Protocol Deviations, Serious Adverse Events (SAEs), Deaths, Confidentiality Breaches, Non-Compliance, and External Events for Emory IRB Approved Studies (*)
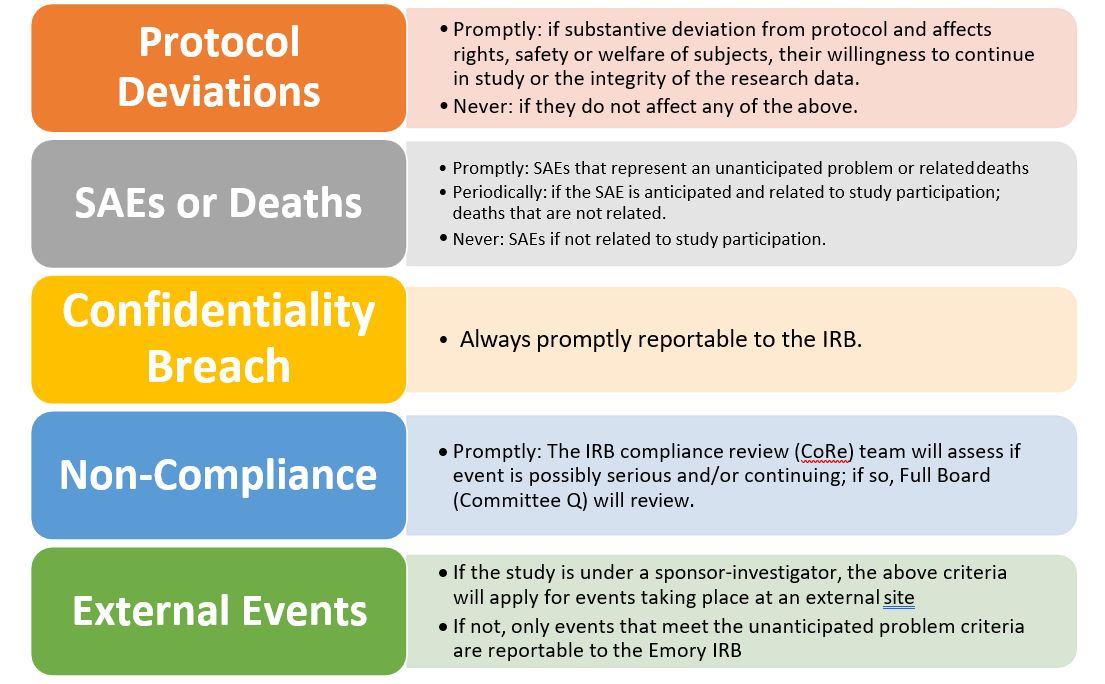
Promptly: 10 business days from the date the PI first learned about the event
Periodically: at continuing review
Unanticipated Problem: event that is unanticipated, related and involving risk to participant or serious.
(*)Studies approved by an External IRB: See Collaborative Page.
VA Guidance and Forms
For information about the reporting procedure for studies conducted at the Atlanta VA, please reference this guidance: VA Office of Research & Development Page and Atlanta VA Research Page and Atlanta VA Policy Documents (for those with access).